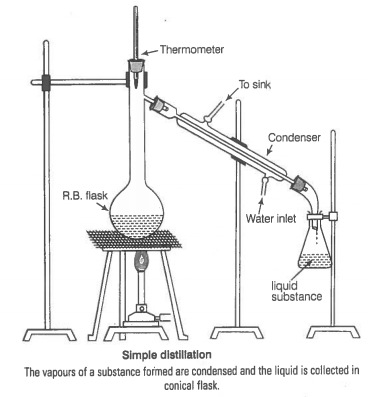
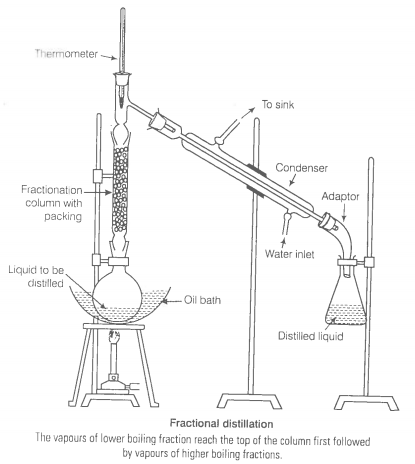
You have a mixture of three liquids A, B and C. There is a large difference in the boiling points of A and rest of the two liquids i.e., B and C. Boiling point of liquids B and C are quite close. Liquid A boils at a higher temperature than B and C and boiling point B is lower than C. How will you separate the components of the mixture. Draw a diagram showing set up of the apparatus for the process.

© 2026 GoodEd Technologies Pvt. Ltd.