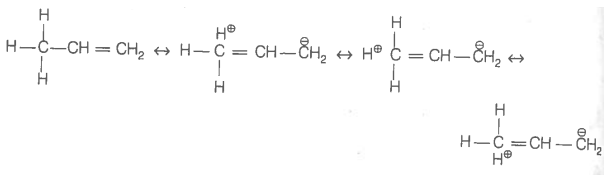
Hyperconjugation involves delocalisation of _____.
1. electrons of carbon-hydrogen bond of an alkyl group directly attached to an atom of unsaturated system.
2. electrons of carbon-hydrogen bond of alkyl group directly attached to the positively charged carbon atom.
3. -electrons of carbon-carbon bond
4. lone pair of electrons.

© 2026 GoodEd Technologies Pvt. Ltd.