Question 12.21: Discuss the chemistry of Lassaigne’s test.
The cyanide, sulphide, and halide of sodium formed are extracted from the fused mass by boiling it in distilled water. The extract so obtained is called Lassaigne’s extract. This Lassaigne’s extract is then tested for the presence of nitrogen, sulphur, halogens, and phosphorous.
(a) Test for nitrogen
Chemistry of the test
In the Lassaigne’s test for nitrogen in an organic compound, the sodium fusion extract is boiled with iron (II) sulphate and then acidified with sulphuric acid. In the process, sodium cyanide first reacts with iron (II) sulphate and forms sodium hexacyanoferrate (II). Then,on heating with sulphuric acid, some iron (II) gets oxidised to form iron (III) hexacyanoferrate (II), which is Prussian blue in colour. The chemical equations involved in the reaction can be represented as
(b) Test for sulphur
Chemistry of the test
In the Lassaigne’s test for sulphur in an organic compound, the sodium fusion extract is acidified with acetic acid and then lead acetate is added to it. The precipitation of lead sulphide, which is black in colour, indicates the presence of sulphur in the compound.
(ii) Lassaigne's extract+Sodium nitroprusside Violet colour
Chemistry of the test
The sodium fusion extract is treated with sodium nitroprusside. Appearance of violet colour also indicates the presence of sulphur in the compound.
If in an organic compound, both nitrogen and sulphur are present, then instead of NaCN, formation of NaSCN takes place.
Na + C + N + S → NaSCN
This NaSCN (sodium thiocyanate) gives a blood red colour. Prussian colour is not formed due to the absence of free cyanide ions.
(c) Test for halogens
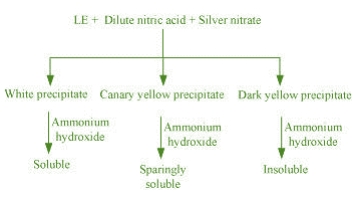
Chemistry of the test
In the Lassaigne’s test for halogens in an organic compound, the sodium fusion extract is acidified with nitric acid and then treated with silver nitrate.
If nitrogen and sulphur both are present in the organic compound, then the Lassaigne’s extract is boiled to expel nitrogen and sulphur, which would otherwise interfere in the test for halogens.