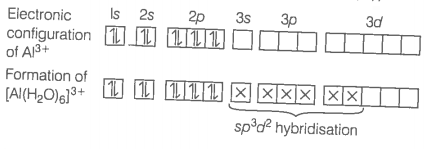
When is treated with water, it hydrolysis and forms on whereas in acidified aqueous solution forms ion. Explain what is the hybridisation or boron and aluminium in these species ?

© 2026 GoodEd Technologies Pvt. Ltd.