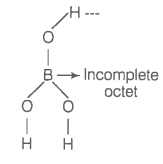
Boric acid is an acid because its molecule
1. Contains replaceable H+ ion
2. Gives up a proton
3. Accepts OH- from water releasing proton
4. Combines with proton from water molecule

© 2026 GoodEd Technologies Pvt. Ltd.