Account for the following observations.
1. AlCl3 is a Lewis acid.
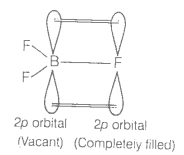
2. Though fluorine is more electronegative than chlorine yet BF3 is a weaker Lewis acid than CI3.
3. PbO2 is a stronger oxidising agent than SnO2.
4. The +1 oxidation state of thallium is more stable than its +3 state.

© 2026 GoodEd Technologies Pvt. Ltd.