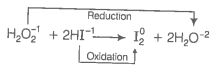
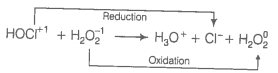
Consider the reactions
(i)
(ii)
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen peroxide is ______.
1. an oxidising agent in both (i) and (ii)
2. an oxidisng agent in (i) and reducing agent in (ii)
3. a reducing agent in (i) and oxidising agent in (ii)
4. a reducing agent in both (i) and (ii)

© 2026 GoodEd Technologies Pvt. Ltd.