9.34 Do you expect different products in solution when aluminium(III) chloride and potassium chloride treated separately with (i) normal water (ii) acidified water, and (iii) alkaline water? Write equations wherever necessary.
In acidified and alkaline water, the ions do not react and remain as such.
Aluminium (III) chloride is the salt of a strong acid (HCl) and weak base [].
Hence, it undergoes hydrolysis in normal water.
In acidified water, H+ ions react with AI forming water and giving ions. Hence, in acidified water, will exist as
ions.
In alkaline water, the following reaction takes place:
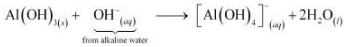

© 2026 GoodEd Technologies Pvt. Ltd.