9.24 Write chemical reactions to show the amphoteric nature of water.
1) Reaction with The reaction takes place as:
In the forward reaction, Hence, it acts as a Lewis base.
2) Reaction with
The reaction takes place as:
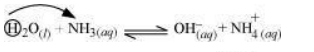
In the forward reaction, denotes its proton to . Hence, it acts as a Lewis acid.
3) Self-ionization of water
In the reaction, two water molecules react as:
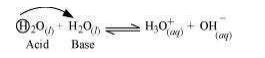

© 2026 GoodEd Technologies Pvt. Ltd.