9.19 Consider the reaction of water with F2 and suggest, in terms of oxidation and reduction, which species are oxidised/reduced.
This is an example of a redox reaction as water is getting oxidized to oxygen, while fluorine is being reduced to fluoride ion.
The oxidation numbers of various species can be represented as:
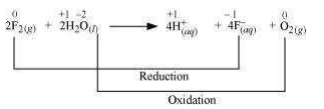
Fluorine is reduced from zero to (– 1) oxidation state. A decrease in oxidation state indicates the reduction of fluorine.
Water is oxidized from (– 2) to zero oxidation state. An increase in oxidation state indicates oxidation of water.

© 2026 GoodEd Technologies Pvt. Ltd.