9.9 What characteristics do you expect from an electron-deficient hydride with respect to its structure and chemical reactions?
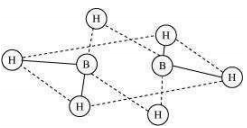
Since these hydrides are electron-deficient, they have a tendency to accept electrons.
Hence, they act as Lewis acids.

© 2026 GoodEd Technologies Pvt. Ltd.