9.8 What do you understand by (i) electron-deficient, (ii) electron-precise, and (iii) electron-rich compounds of hydrogen? Provide justification with suitable examples.
1. Electron-deficient hydrides
2. Electron-precise hydrides
3. Electron-rich hydrides
An electron-deficient hydride has very few electrons, less than that required for representing its conventional Lewis structure e.g. diborane (). In , there are six bonds in all, out of which only four bonds are regular two centered-two electron bonds. The remaining two bonds are three centered-two electron bonds i.e., two electrons are shared by three atoms. Hence, its conventional Lewis structure cannot be drawn. An electron-precise hydride has a sufficient number of electrons to be represented by its conventional Lewis structure e.g. . The Lewis structure can be written as:
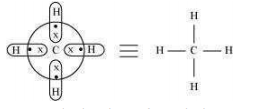
Four regular bonds are formed where two electrons are shared by two atoms.
An electron-rich hydride contains excess electrons as lone pairs e.g. .
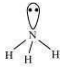
There are three regular bonds in all with a lone pair of electrons on the nitrogen atom.

© 2026 GoodEd Technologies Pvt. Ltd.