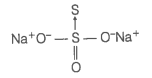
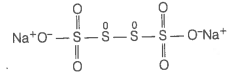The oxidation number of each sulphur atom in the following compounds are given below
(a) Let us consider the structure of .
There is a coordinate bond between two sulphur atoms. The oxidation number of acceptor S-atom is -2. Let, the oxidation number of other S-atom be x.
Therefore, the two sulphur atoms in have -2 and +6 oxidation number.
(b) Let us consider the structure of .
In this structure, two central sulphur atoms have zero oxidation number because electron pair forming the S-S bond remain in the centre. Let, the oxidation number of (remaining S-atoms) S-atom be x.
Therefore, the two central S-atoms have zero oxidation state and two terminal S-atoms have +5 oxidation state each.
(c) Let the oxidation number of S in be x.
(d) Let the oxidation number of S be x.