8.5 Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, Cr2O72– and NO3–. Suggest structure of these compounds. Count for the fallacy.
However, the O.N. of S cannot be +8. S has six valence electrons. Therefore, the O.N. of S cannot be more than +6.
The structure of is shown as follows: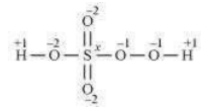
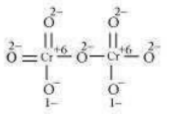
Here, each of the two Cr atoms exhibits the O.N. of +6.
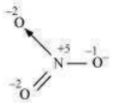
The N atom exhibits the O.N. of +5.

© 2026 GoodEd Technologies Pvt. Ltd.