The oxidation number of each element in the given reaction can be represented as:
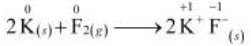
In this reaction, the oxidation number of K increases from 0 in K to +1 in KF i.e., K is oxidized to KF. On the other hand, the oxidation number of F decreases from 0 in F to –1 in KF i.e., F is reduced to KF.
Hence, the above reaction is a redox reaction.
(e) ![]()
The oxidation number of each element in the given reaction can be represented as:
![]()
Here, the oxidation number of N increases from –3 in NH to +2 in NO. On the other hand, the oxidation number of O decreases from 0 in O to –2 in NO and HO i.e., O is reduced. Hence, the given reaction is a redox reaction.
8.4 Fluorine reacts with ice and results in the change:
H2O(s) + F2(g) → HF(g) + HOF(g)
Justify that this reaction is a redox reaction.
Let us write the oxidation number of each atom involved in the given reaction above its symbol as:
Here, we have observed that the oxidation number of F increases from 0 in to +1 in HOF. Also, the oxidation number decreases from 0 in to –1 in HF. Thus, in the above reaction, F is both oxidized and reduced. Hence, the given reaction is a redox reaction.

© 2026 GoodEd Technologies Pvt. Ltd.