8.3 Justify that the following reactions are redox reactions:
(a) CuO(s) + H2(g) → Cu(s) + H2O(g)
(b) Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)
(c) 4BCl3(g) + 3LiAlH4(s) → 2B2H6(g) + 3LiCl(s) + 3 AlCl3 (s)
(d) 2K(s) + F2(g) → 2K+F– (s)
(e) 4 NH3(g) + 5 O2(g) → 4NO(g) + 6H2O(g)
(a) CuO(s)+H(g)→Cu(s)+HO(g)
Let us write the oxidation number of each element involved in the given reaction as:
![]()
Here, the oxidation number of Cu decreases from +2 in CuO to 0 in Cu i.e., CuO is reduced to Cu. A/so, the oxidation number of H increases from 0 in H to +1 in HO i.e., H is oxidized to HO. Hence, this reaction is a redox reaction.
(b) ![]()
Let us write the oxidation number of each element in the given reaction as:
![]()
Here, the oxidation number of Fe decreases from +3 in FeO to 0 in Fe i.e., FeO is reduced to Fe. On the other hand, the oxidation number of C increases from +2 in CO to +4 in CO i.e., CO is oxidized to CO. Hence, the given raaction is a redox reaction.
(c) ![]()
The oxidation number of each element in the given reaction can be represented as:
![]()
In this reaction, the oxidation number of B decreases from +3 in BCl to –3 in BH. i.e., BCl is reduced to BH. Also, the oxidation number of H increases from –1 in LiAlH to +1 in BH i.e., LiAlH4 is oxidized to BH. Hence, the given reaction is a redox reaction.
(d) ![]()
The oxidation number of each element in the given reaction can be represented as:
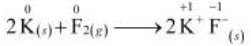
In this reaction, the oxidation number of K increases from 0 in K to +1 in KF i.e., K is oxidized to KF. On the other hand, the oxidation number of F decreases from 0 in F to –1 in KF i.e., F is reduced to KF.
Hence, the above reaction is a redox reaction.
(e) ![]()
The oxidation number of each element in the given reaction can be represented as:
![]()
Here, the oxidation number of N increases from –3 in NH to +2 in NO. On the other hand, the oxidation number of O decreases from 0 in O to –2 in NO and HO i.e., O is reduced. Hence, the given reaction is a redox reaction.

© 2026 GoodEd Technologies Pvt. Ltd.