7.19 A sample of pure PCl5 was introduced into an evacuated vessel at 473 K. After equilibrium was attained, concentration of PCl5 was found to be
0.5 × 10–1 mol L–1. If value of Kc is 8.3 × 10–3, what are the concentrations of PCl3 and Cl2 at equilibrium?
PCl5 (g) 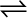 PCl3 (g) + Cl2(g)
PCl3 (g) + Cl2(g)
Let the concentrations of both at equilibrium be x mol. The given reaction is:
It is given that of the value of the equilibrium constant, .
Now we can write the expression for equilibrium as:
Therefore, at equilibrium,

© 2026 GoodEd Technologies Pvt. Ltd.