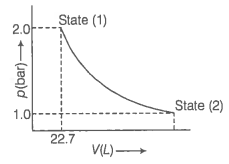
1.0 mol of monoatomic ideal gas is expanded from state (1) to state (2) as shown in figure. Calculate the work done for the expansion of gas from state (1) to state (2) at 298 K.

© 2026 GoodEd Technologies Pvt. Ltd.