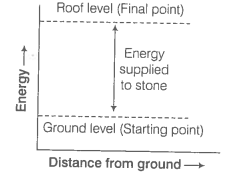
Represent the potential energy/enthalpy change in the following processes graphically.
1. Throwing a stone from the ground to roof.
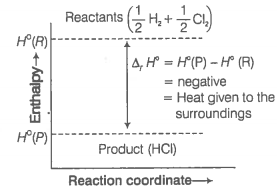
2.
In which of the processes potential energy/enthalpy change is contributing factor to the spontaneity?

© 2026 GoodEd Technologies Pvt. Ltd.