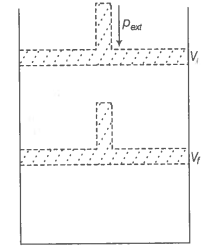
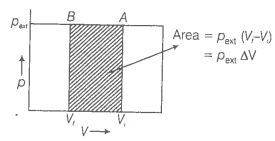
What will be the work done on an ideal gas enclosed in a cylinder, when it is compressed by a constant external pressure, pext in a single step as shown in figure? Explain graphically.

© 2026 GoodEd Technologies Pvt. Ltd.