The lattice enthalpy of an ionic compound is the enthalpy change which occurs when one mole of an ionic compound dissociates into its ions in gaseous state. For the reaction
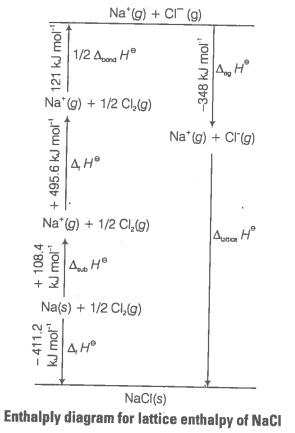
Since, it is impossible to determine lattice enthalpies directly by experiment, we use an indirect method where we construct an enthalpy diagram called a Born-Haber cycle.
Let us now calculate the lattice enthalpy of by following steps given below
(i) Sublimation of sodium metal,
(ii) The ionisation of sodium atoms, ionisation enthalpy
(iii) The dissociation of chlorine, the reaction enthalpy is half the bond dissociation enthalpy
(iv) electron gained by chlorine atoms. The electron gain enthalpy,
(v)
The sequence of steps is shown in given figure and is known as Born-Haber cycle. The importance of the cycle is that, the sum of the enthalpy changes round a cycle is zero.
Applying Hess's law, we get