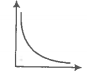
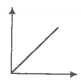
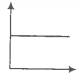
Match the following graphs of an ideal gas with their coordinates.
|
Graphical representation |
X and Y coordinates |
|
A. |
1. pV vs V |
|
B. |
2. p vs V |
|
C. |
3. p vs |
Codes
| Options: | A | B | C |
| 1. | 2 | 3 | 1 |
| 2. | 1 | 2 | 3 |
| 3. | 3 | 2 | 1 |
| 4. | 1 | 3 | 1 |

© 2026 GoodEd Technologies Pvt. Ltd.