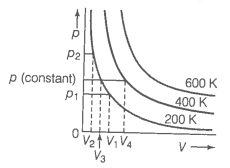
The variation of pressure with volume of the gases at different temperatures can be graphically represented as shown in figure. On the basis of this graph answer the following questions.
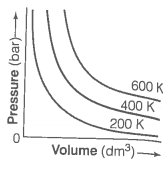
1. How will the volume of a gas change if its pressure is increased at constant temperature?
2. At a constant pressure, how will the volume of a gas change if the temperature is increased from 200 K to 400 K?

© 2026 GoodEd Technologies Pvt. Ltd.