One of the assumptions of kinetic theory of gases is that there is no force of attraction between the molecules of a gas.
State and explain the evidence that shows that the assumption is not applicable for real gases.
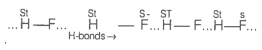

© 2026 GoodEd Technologies Pvt. Ltd.