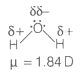
Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of dipoles possess 'partial charges'.
The partial charge will be-
1. More than unit electronic charge.
2. Equal to unit electronic charge.
3. Less than unit electronic charge.
4. Double the unit electronic charge.

© 2026 GoodEd Technologies Pvt. Ltd.