1. Discuss the concept of hybridisation. What are its different types in a carbon atom?
2. What is the type of hybridisation of carbon atoms marked with star?
(i) 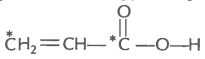
(ii) 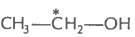
(iii) 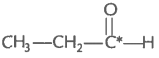
(iv) 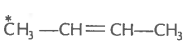
(v) 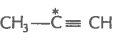
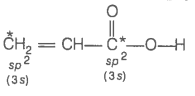
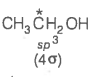
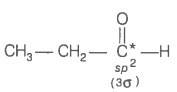
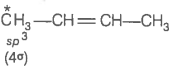
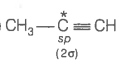

© 2026 GoodEd Technologies Pvt. Ltd.