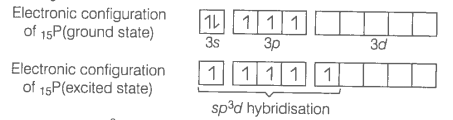
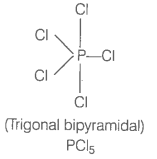
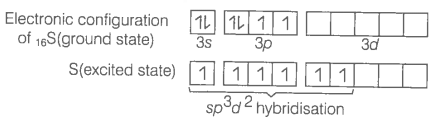
Describe hybridisation in the case of PCl5 and SF6. The axial bonds are longer as compared to equatorial bonds in PCl5 wherea in SF6 both axial bonds and equatorial bonds have the same bond length. Explain.

© 2026 GoodEd Technologies Pvt. Ltd.