Valence bond theory (VBT) was introduced by Heitler and London (1927) and developed further by Pauling and other. VBT is based on the knowledge of atomic orbitals, electronic configurations of elements, the overlap criteria of atomic orbitals, the hybridisation of atomic orbitals and the principles of variation and superposition.
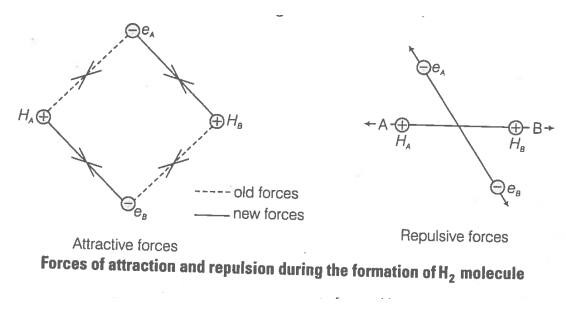
Consider two hydrogen atoms A and B approaching each other having nuclei NA and NB and electrons present in them are represented by eA and eB. When the two atoms are at large distance from each other, there is no interaction between them.
As these two atoms approach each other, new attractive and repulsive forces begin to operate.
Attractive forces arise between
(i) nucleus of one atom and its own electron
i.e., NA-eA and NB-eB
(ii) nucleus of one atom and electron of other atom
i.e., NA-eB, NB-eA
Similarly, repulsive forces arise between
(i) electrons of two atoms like eA-eB
(ii) nuclei of two atoms like NA-NB
Attractive forces tend to bring the two atoms close to each other whereas repulsive forces tend to push them apart.
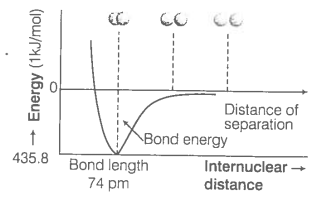
Experimentally, we have been found that the magnitude of new attractive force is more than the new repulsive forces. As a result two atoms approach each other and potential energy decreases.
Hence, a stage is reached where the net force of attraction balances the force of repulsion and system acquires minimum energy. At this stage, two H-atoms are said to be bonded together to form a stable molecule having the bond length of 74 pm.
Since, the energy gets released when the bond is formed between two hydrogen atoms, the hydrogen molecule is more stable than that of isolated hydrogen atoms.
The energy so released is called as bond enthalpy, which is corresponding to minimum in the curve depicted in the given figure. Conversely 435.8 kJ of energy is required to dissociate one mole of H2 molecule.
The potential energy curve for the formation of H2 molecule as a function of internuclear distance of the H-atoms. The minimum in the curve corresponds to the most stable state or H2.