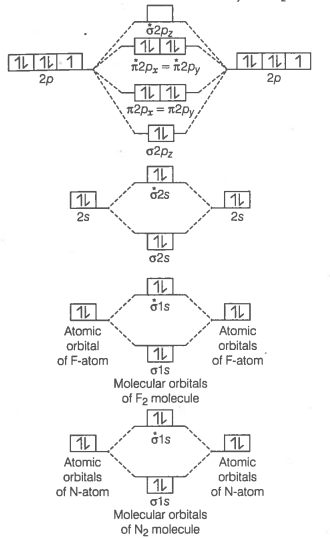
Formation of N2 molecule Electronic configuration of N-atom
molecule
Bond order
Bond order value of 3 means that N2 contains a triple bond.
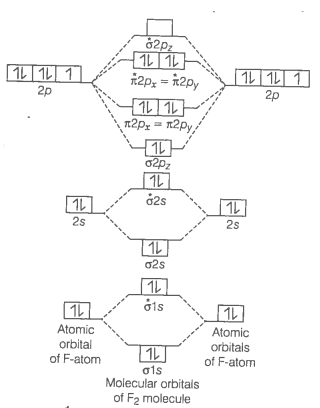
Formation of F2 molecule
molecule
Bond order
Bond order value 1 means that F2 contains single bond.
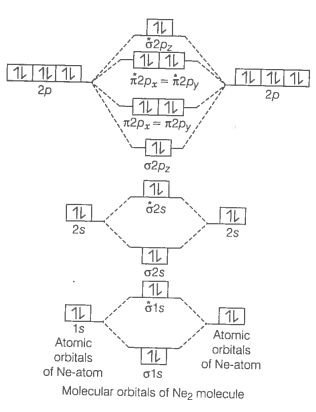
Formation of Ne2 molecule
Ne2 molecule
Bond order
Bond order value zero means that there is no formation of bond between two Ne-atoms.
Hence, Ne2 molecule does not exist.