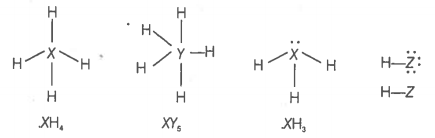
Elements X, Y and Z have 4, 5 and 7 valence electrons respectively.
1. Write the molecular formula of the compounds formed by these elements individually with hydrogen.
2. Which of these compounds will have the highest dipole moment?

© 2026 GoodEd Technologies Pvt. Ltd.