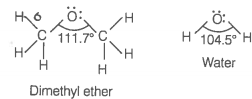
In both water and dimethyl ether ![]() , oxygen atom is central atom, and has the same hybridisation, yet they have different bond angles. Which one has greater bond angle? Give reason.
, oxygen atom is central atom, and has the same hybridisation, yet they have different bond angles. Which one has greater bond angle? Give reason.

© 2026 GoodEd Technologies Pvt. Ltd.