Structures of molecules of two compounds are given below.
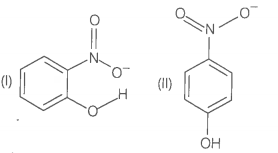
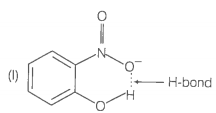
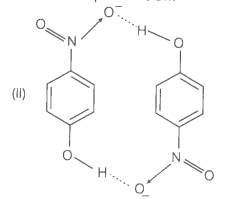
1. Which of the two compounds will have intermolecular hydrogen bonding and which compound is expected to show intramolecular hydrogen bonding?
2. The melting point of compound depends on, among other things, the extent of hydrogen bonding. On this basis explain which of the above two compounds will show higher melting point?
3. Solubility of compounds in water depends on power to form hydrogen bonds with water. Which of the above compounds will form hydrogen bond with easily and be more soluble in it?

© 2026 GoodEd Technologies Pvt. Ltd.