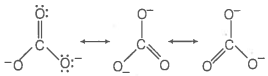
Which of the following statements are correct about ?
1. The hybridisation of central atom is sp3
2. Its resonance structure has one C-O single bond and two C=O double bonds
3. The average formal charge on each oxygen atom is 0.67 units
4. All C-O bond lengths are equal

© 2026 GoodEd Technologies Pvt. Ltd.