4.26 Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
Boron atom in is sp hybridized. The orbital picture of boron in the excited state can
be shown as:
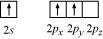
Nitrogen atom in is sp hybridized. The orbital picture of nitrogen can be represented
as:
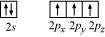
After the reaction has occurred, an adduct FBNH is formed as hybridization of ‘B’ changes to sp. However, the hybridization of ‘N’ remains intact.

© 2026 GoodEd Technologies Pvt. Ltd.