4.25 Describe the change in hybridisation (if any) of the Al atom in the following reaction.
The valence orbital picture of aluminium in the ground state can be represented as:
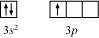
The orbital picture of aluminium in the excited state can be represented as:
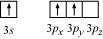
Hence, it undergoes sp hybridization to give a trigonal planar arrangement (in AlCl). To
form
, the empty 3p orbital also gets involved and the hybridization changes from sp
to sp . As a result, the shape gets changed to tetrahedral.

© 2026 GoodEd Technologies Pvt. Ltd.