4.24 What is meant by hybridisation of atomic orbitals? Describe the shapes of sp, , hybrid orbitals.
Hybridization is defined as an intermixing of a set of atomic orbitals of slightly different
energies, thereby forming a new set of orbitals having equivalent energies and shapes.
For example, one 2s-orbital hybridizes with two 2p-orbitals of carbon to form three new
hybrid orbitals.
These hybrid orbitals have minimum repulsion between their electron pairs and thus, are
more stable. Hybridization helps indicate the geometry of the molecule.
Shape of sp hybrid orbitals: sp hybrid orbitals have a linear shape. They are formed by
the intermixing of s and p orbitals as:
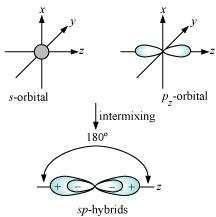
Shape of hybrid orbitals:
sp hybrid orbitals are formed as a result of the intermixing of one s-orbital and two
2porbitals. The hybrid orbitals are oriented in a trigonal planar arrangement as:
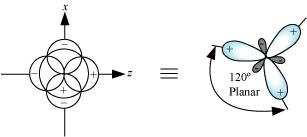
Shape of hybrid orbitals:
Four sp hybrid orbitals are formed by intermixing one s-orbital with three p-orbitals.
The four sp hybrid orbitals are arranged in the form of a tetrahedron as:
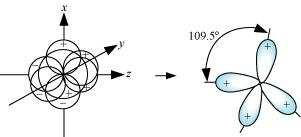

© 2026 GoodEd Technologies Pvt. Ltd.