4.10 Define the bond length.
Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms
in a molecule.
Bond lengths are expressed in terms of Angstrom (10–10 m) or picometer
(10–12 m) and are measured by spectroscopic X-ray diffractions and electron-diffraction
techniques.
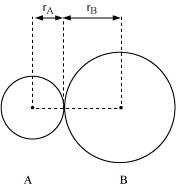
In an ionic compound, the bond length is the sum of the ionic radii of the constituting
atoms (d = ). In a covalent compound, it is the sum of their covalent radii (d = )

© 2026 GoodEd Technologies Pvt. Ltd.