2.66 Indicate the number of unpaired electrons in : (a) P, (b) Si, (c) Cr, (d) Fe and (e) Kr.
(a) Phosphorus (P):
Atomic number = 15
The electronic configuration of P is:
The orbital picture of P can be represented as: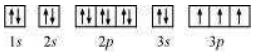
From the orbital picture, phosphorus has three unpaired electrons.
(b) Silicon (Si):
Atomic number = 14
The electronic configuration of Si is:
The orbital picture of Si can be represented as: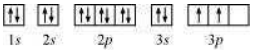
From the orbital picture, silicon has two unpaired electrons.
(c) Chromium (Cr):
Atomic number = 24
The electronic configuration of Cr is:
The orbital picture of chromium is: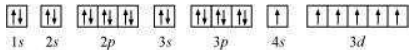
From the orbital picture, chromium has six unpaired electrons.
(d) Iron (Fe):
Atomic number = 26
The electronic configuration is:
The orbital picture of chromium is: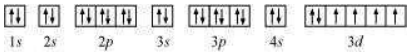
From the orbital picture, iron has four unpaired electrons.
(e) Krypton (Kr):
Atomic number = 36
The electronic configuration is:
The orbital picture of krypton is: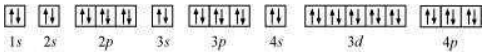
Since all orbitals are fully occupied, there are no unpaired electrons in krypton.

© 2026 GoodEd Technologies Pvt. Ltd.