2.3 How many neutrons and protons are there in the following nuclei?
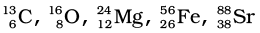
:
Atomic mass = 13
Atomic number = Number of protons = 6
Number of neutrons = (Atomic mass) – (Atomic number)
= 13 – 6 = 7
:
Atomic mass = 16
Atomic number = 8
Number of protons = 8
Number of neutrons = (Atomic mass) – (Atomic number)
= 16 – 8 = 8
:
Atomic mass = 24
Atomic number = Number of protons = 12
Number of neutrons = (Atomic mass) – (Atomic number)
= 24 – 12 = 12
:
Atomic mass = 56
Atomic number = Number of protons = 26
Number of neutrons = (Atomic mass) – (Atomic number)
= 56 – 26 = 30
:
Atomic mass = 88
Atomic number = Number of protons = 38
Number of neutrons = (Atomic mass) – (Atomic number)
= 88 – 38 = 50

© 2026 GoodEd Technologies Pvt. Ltd.