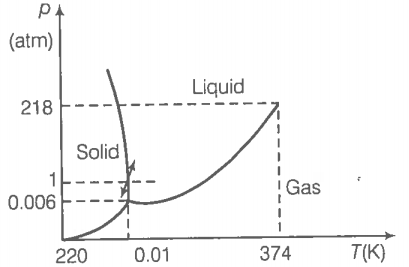
During summers in India, one of the common practice to keep cool is to make ice balls of crushed ice, dip it in flavoured sugar syrup and sip it. For this, a stick is inserted into crushed ice and is squeezed into the palm to make it into the ball. Equivalently in winter in those areas where it snows, people make snowballs and throw around. Explain the formation of a ball out of crushed ice or snow in the light of PT diagram of water.

© 2026 GoodEd Technologies Pvt. Ltd.