Refer to the plot of temperature versus time (figure) showing the changes in the state of ice on heating (not to scale).
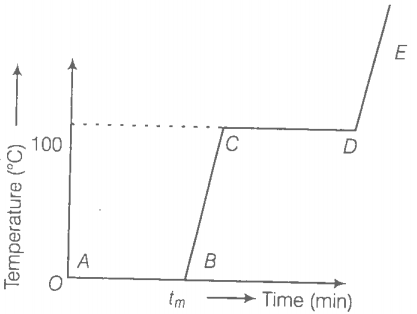
(a) The region AB represents the ice and water in thermal equilibrium.
(b) At B, the water starts boiling.
(c) At C, all the water gets converted into steam.
(d) C to D represents water and steam in equilibrium at boiling point.
Choose the correct alterative/s:
1. (b, c)
2. (a, d)
3. (c, d)
4. (b, d)

© 2026 GoodEd Technologies Pvt. Ltd.