12.9 A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in Fig. (12.13)
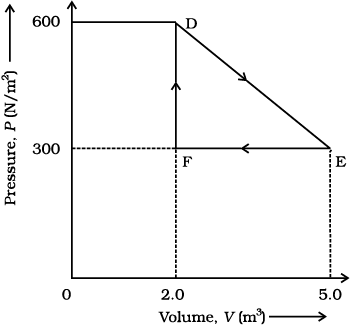 Fig. 12.13
Fig. 12.13Its volume is then reduced to the original value from E to F by an isobaric process. Calculate the total work done by the gas from D to E to F.
Total work done by the gas from D to E to F = Area of ΔDEF
Area of ΔDEF =
Area of ΔDEF = = 450 J
Therefore, the total work done by the gas from D to E to F is 450 J.

© 2026 GoodEd Technologies Pvt. Ltd.