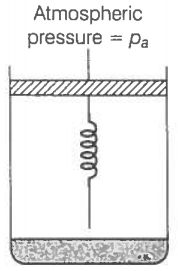
Consider one mole of a perfect gas in a cylinder of the unit cross-section with
a piston attached (figure). A spring (spring constant k) is attached (unstretched length L) to the piston and to the bottom of the cylinder. Initially, the spring is unstretched and the gas is in equilibrium. A certain amount of heat Q is supplied to the gas causing an increase of value from to .
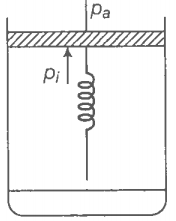
(a) What is the initial pressure of the system?
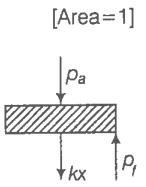
(b) What is the final pressure of the system?
(c) Using the first law of thermodynamics, write down the relation between Q, , , and k.

© 2026 GoodEd Technologies Pvt. Ltd.