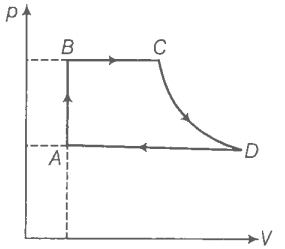
Hint: Use the first law of thermodynamics.
(a) Step 1: Find the heat exchanged by the engine in the process AB.
For process AB,
Volume is constant, hence work done dW = 0
Now, by the first law of thermodynamics,
Heat exchanged
(b) Step 2: Find the heat exchanged by the engine in the process BC.
For process BC, P=constant
Heat exchanged and
(c) Step 3: Find the heat exchanged by the engine in the process CD.
Because CD is adiabatic, dQ = the heat exchanged = 0
(d) Step 4: Find the heat exchanged by the engine in process AB.
DA involves compression of gas from to at constant pressure .
Heat exchanged can be calculated in a similar way as .
Hence,