Hint: Use the first law of thermodynamics.
(a) Step 1: Find if the work is zero and dU > 0 in any process.
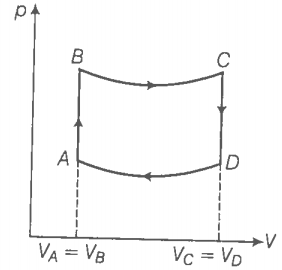
For the process AB,
dV = 0 dW = 0 ( volume is constant)
dQ = dU+ dW = dU
dQ = dU = Change in internal energy.
Hence, in this process, the heat supplied is utilised to increase the internal energy of the system.
Since, pT, in isochoric process,. So the temperature increases with increases of pressure in process AB which in turn increases the internal energy of the system ie., dU > 0. This implies that dQ > 0. So the heat is supplied to the system in process AB.
(b) Step 2: Find if the work is zero and dU < 0 in any process.
For the process CD, the volume is constant but the pressure decreases. Hence, the temperature also decreases. So the heat is given to the surroundings.
(c) Step 3: Find the work done by the engine in one cycle.
To calculate the work done by the engine in one cycle, we calculate the work done in each part separately.
Similarly, [ BC and DA are adiabatic processes]
B and C lie on adiabatic curve BC.
Similarly,
Total work done by the engine in one cycle ABCDA,
(d) Step 4: Find the efficiency of the engine.
The heat supplied during the process AB,
The efficiency of the engine,