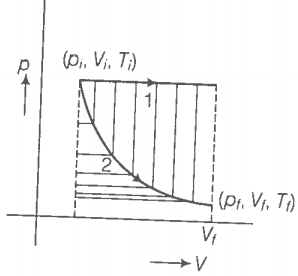
The initial state of a certain gas is (). It undergoes expansion till its volume becomes . Consider the following two cases:
(a) the expansion takes place at a constant temperature.
(b) the expansion takes place at constant pressure.
Plot the p-V diagram for each case. In which of the two cases, is the work done by the gas more?

© 2026 GoodEd Technologies Pvt. Ltd.