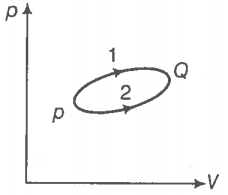
A system goes from P to Q by two different paths in the P-V diagram as shown in the figure. Heat given to the system in path 1 is 1000 J. The work done by the system along path 1 is more than path 2 by 100 J. What is the heat exchanged by the system in path 2?

© 2026 GoodEd Technologies Pvt. Ltd.