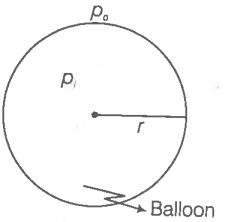
Hint: Apply the concept of excess pressure.
Step 1: Find the excess pressure inside the balloon.
Let the pressure inside the balloon be and the outside pressure be , then excess
pressure is
where S = Surface tension
r= radius of the balloon
Considering the air to be an ideal gas, where, V is the volume of the air inside the balloon, is the number of moles inside the balloon and is the temperature inside the balloon, and where V is the volume of the air displaced and is the number of moles displaced and is the temperature outside.
So.
where is the mass of air inside and is the molar mass of air.
and
where is the mass of air outside that has been displaced.
Step 2: Find the weight lifted by the balloon.
If w is the load it can raise, then,
w +
As in atmosphere are present.
The molar mass of air,
Weight raised by the balloon,
Masslifted by the balloon,