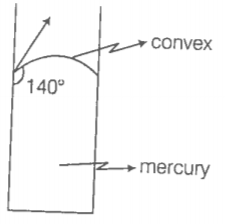
The angle of contact at the interface of the water glass is \(0^{\circ},\) ethyl-alcohol glass is \(0^{\circ},\) mercury-glass is \(140^{\circ}\) and methyl iodide-glass is \(30^{\circ}.\) A glass capillary is put in a trough containing one of these four liquids is observed that the meniscus is convex. The liquid in the trough is:
1. water
2. ethyl alcohol
3. mercury
4. methyl iodide

© 2026 GoodEd Technologies Pvt. Ltd.