10.22 A manometer reads the pressure of a gas in an enclosure as shown in Fig. 10.25 (a). When a pump removes some of the gas, the manometer reads as in Fig. 10.25 (b). The liquid used in the manometers is mercury and the atmospheric pressure is 76 cm of mercury.
(a) Give the absolute and gauge pressure of the gas in the enclosure for cases (a) and (b), in units of cm of mercury.
(b) How would the levels change in case (b) if 13.6 cm of water (immiscible with mercury) is poured into the right limb of the manometer? (Ignore the small change in the volume of the gas).
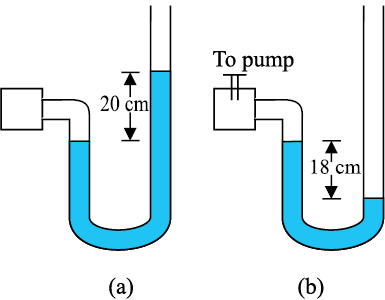
Fig. 10.25
(a)
For figure (a):
Atmospheric pressure, P0 = 76 cm of Hg
Gauge pressure = difference between the levels of mercury in the two limbs = 20 cm of Hg
Absolute pressure = Atmospheric pressure + Gauge pressure = 76 + 20 = 96 cm of Hg
For figure (b):
Gauge pressure = difference between the levels of mercury in the two limbs = –18 cm
Absolute pressure = Atmospheric pressure + Gauge pressure= 76 cm – 18 cm = 58 cm
(b)
The relative density of mercury = 13.6
A column of 13.6 cm of water = 1 cm of mercury
Let h be the difference between the levels of mercury in the two limbs.
The pressure in the right limb is given as:
Atmospheric pressure + 13.6 cm of water = Atmospheric pressure + 1 cm of Hg = 76 + 1 = 77 cm of Hg
The mercury column will rise in the left limb.
Pressure in the left limb,
,

© 2026 GoodEd Technologies Pvt. Ltd.